A New Clinical Research Study for People with Retinitis Pigmentosa
Welcome to the Aldeyra Therapeutics Retinitis Pigmentosa Study!
The goal of this study is to see if the investigational drug ADX-2191 is safe and effective in patients with retinitis pigmentosa (RP) due to confirmed rhodopsin mutations.
Participants who qualify will be asked to complete 15 study visits over 13 months, including the procedures and follow-up appointments.

You may be eligible for this clinical trial if:
You are 21 years old or older
You have been diagnosed with retinitis pigmentosa (RP)
You are willing to attend up to 15 study visits in a 13-month period
Other criteria may apply
Eligible study participants will:
- Be connected with their nearest research clinic
- Receive study information and learn more about RP
- Undergo health assessments and study procedures at no cost to them
- Meet with a research team 15 times over the course of 13 months
- Support research and the development of new treatments
About Retinitis Pigmentosa
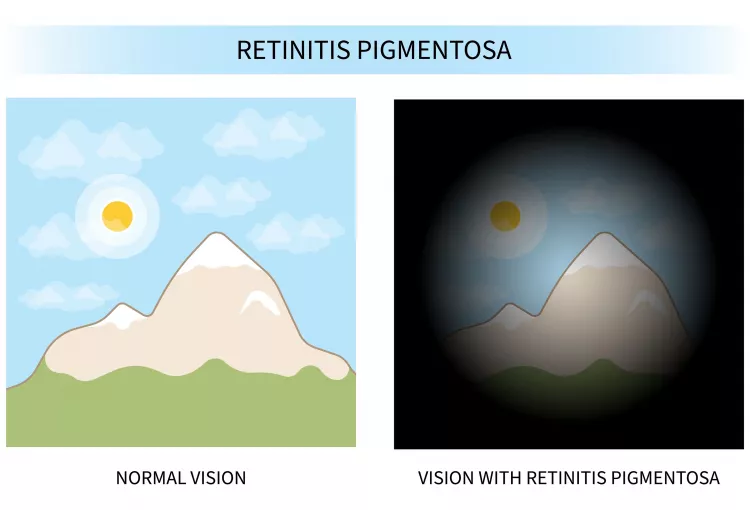
Retinitis pigmentosa (RP) is a group of rare eye diseases that affect the retina (the light-sensitive layer of tissue in the back of the eye). RP makes cells in the retina break down slowly over time, causing vision loss.
RP is a genetic disease that people are born with. Symptoms usually start in childhood, and most people eventually lose most of their sight. While there is currently no cure for RP, vision aids and rehabilitation can help patients make the most of their vision. In some cases, medication or surgery can address compounding issues like cataracts.
Scientists are continually researching new approaches to treating RP, but they cannot do it alone. ADX-2191 represents an investigational approach for the treatment of people living with retinitis pigmentosa.
Did You Know?
All prescription drugs that are taken today were once studied in clinical trials. Scientists cannot study ground-breaking therapies without the help of volunteers. New medications cannot be prescribed unless they are found to be safe and effective in clinical trials.

A large number of experimental drugs never get a chance to be researched because scientists are unable to find enough volunteers for the clinical trial.

Any qualified, experienced medical doctor can become a trial doctor, even your family physician or eye doctor.

Trial sites can be any hospital, medical clinic, or doctor’s office where trial-related assessments take place.

You do not have to pay or have health insurance to sign up for a clinical trial. Volunteers often receive compensation for participating in a clinical trial.
Frequently Asked Questions
What is a research study?
Medical research studies (or clinical trials) help doctors and scientists learn about experimental drugs, including how well they work and how safe they are. Clinical trials may study the experimental drug (or study drug) by itself, compare it to an approved medication, or placebo.
What should I expect if I join a clinical trial?
Every clinical trial is different, so it is important to always read all the information that is provided by the trial doctor and research staff. The trial participants will always be informed of the procedures that they can expect to undergo before they decide to join the clinical trial. Participation in a clinical trial is usually broken down into three separate stages: Screening, Study treatment, and Follow-up.
- Screening: The purpose of the screening period is to make sure participants are suitable to take part in the trial.
- Treatment: If a participant is deemed eligible, they will officially be enrolled in the clinical trial and may receive the study drug. More information will be provided in your consent form.
- Follow-up: Participants will complete one last visit after they are done receiving the study drug.
What assessments will be performed during this trial?
There are common assessments that are performed during most clinical studies. Here is what you can expect during the ADX-2191 study:
1. Read and sign the Informed Consent Form (ICF)
- The ICF explains the study in more detail. The study doctor will explain the information in the ICF and answer any questions.
- You must sign the ICF before receiving any study treatment or assessments.
- Remember that taking part in the study is your choice. You can stop at any time.
2. Confirm that you can join the study
- Participants who would like to join the study will undergo screening to ensure they are a good fit for the study. Study assessments/procedures may include:
- Demographics: You will be asked to provide personal information, including date of birth, gender, ethnic origins, and race (demographics).
- Medical History: You will be asked about your past and present health conditions, including retinitis pigmentosa. You must provide accurate and complete information about your medical history and present health conditions.
- Physical Exam: You will have physical examinations, including a full ophthalmic exam, as well as safety laboratory assessments.
3. Receive Treatment
If they meet the study requirements, they will be enrolled and receive the experimental treatment. All eligible participants will be randomized into 2 groups, like flipping a coin, and receive either a full dose or a low dose of ADX-2191.
4. Visit the study clinic
Eligible participants will be asked to complete 15 study visits during a 13-month period.
How long will this trial last?
Your participation in this study is expected to last 13 months. The trial doctor will always tell you how long the trial is before you decide to join.
Do I have to pay to join a clinical trial?
You do not have to pay or have health insurance to sign up for a clinical trial. The trial drug and trial-related procedures are provided to participants at no charge to them or their health insurance company. Clinical trials often provide participants compensation (or a stipend) for their time and effort.

