
The Crucial Role of Real-World Data and Real-World Evidence in Advancing Clinical Research
In the realm of healthcare and medical advancements, the terms real-world data (RWD) and real-world evidence (RWE) have emerged as pivotal concepts that are transforming the terrain of clinical research. As the healthcare industry becomes increasingly data-driven, these terms can significantly enhance our understanding of treatments, interventions, and patient outcomes in real-world settings.
Understanding RWD and RWE
RWD refers to data collected from various sources outside the confines of traditional clinical trials. This data is derived from electronic health records, insurance claims, wearable devices, patient registries, and even social media platforms. Unlike data from controlled clinical trials, RWD offers insights into how treatments and other interventions perform in everyday clinical practice, encompassing a much broader patient population.
RWE, on the other hand, refers to insights gleaned from the analysis of RWD. It provides valuable information about the efficacy, safety, and economic impacts of interventions in real-world settings. RWE plays an essential role in bridging the gap between controlled clinical trials and practical application.
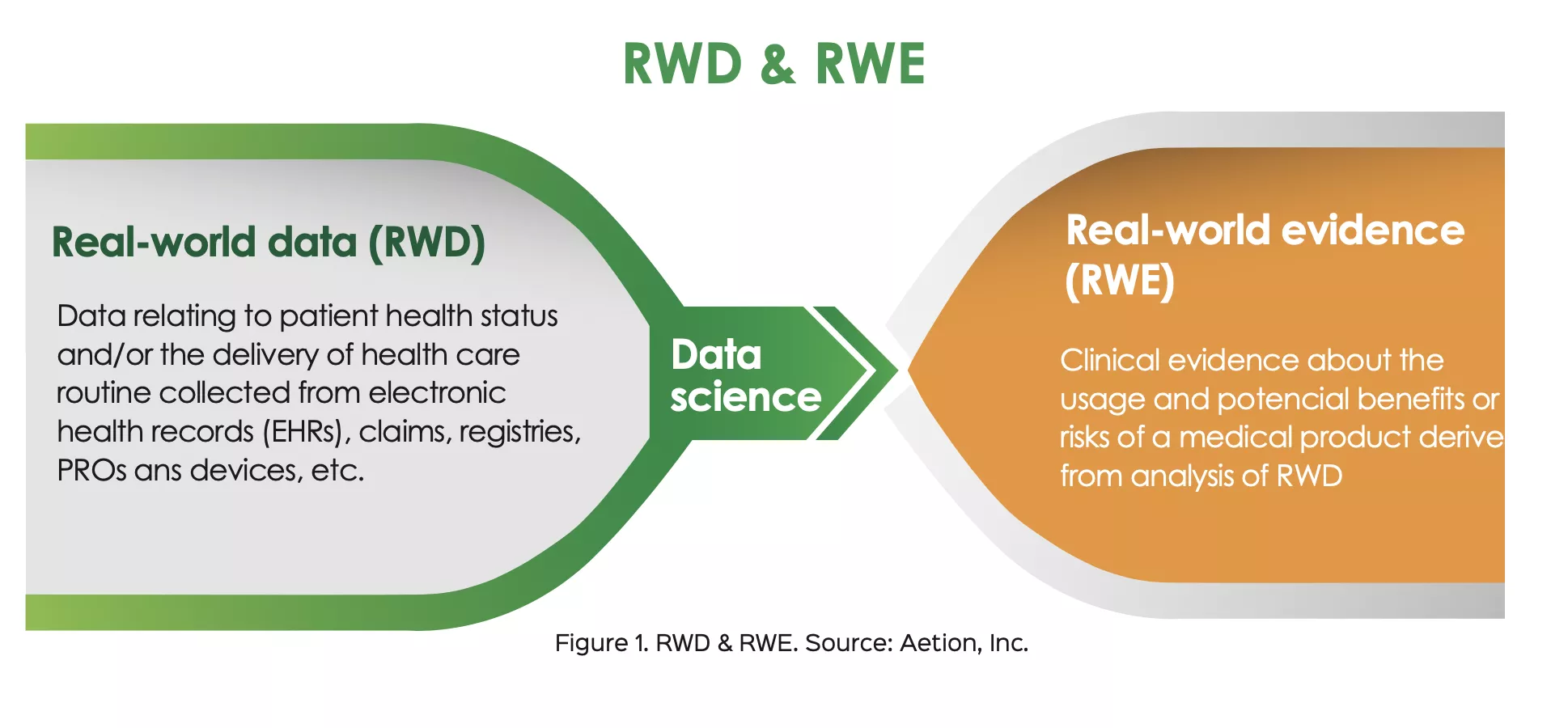
The importance of RWD and RWE in clinical research
RWD and RWE are critical components of successful, productive clinical research. Some of the reasons for this include:
- Correcting underrepresentation of certain demographics. RWD captures data from a more diverse patient pool, offering insights into how interventions work across different age groups, ethnicities, and co-morbidities.
- RWD allows researchers to track patient outcomes over a much longer period, providing a comprehensive view of the long-term effects of treatments.
- Trials for rare diseases are challenging due to small patient populations. RWD enables researchers to accumulate data from a larger variety of sources, helping them understand the natural history of these diseases and evaluate the effectiveness of treatments.
- Once a drug or medical device is approved and enters the market, its performance in real-world scenarios becomes crucial. RWE aids in post-marketing surveillance, identifying potential safety issues that might not have been evident in controlled trials.
- RWE provides insights into the cost-effectiveness of treatments, helping healthcare systems make informed decisions about reimbursement and market access. This information is pivotal in ensuring that patients have access to the most effective interventions.
- RWD analysis can unveil alternative uses for existing drugs. This is particularly valuable in cases where a drug designed for one condition shows promise in treating another, potentially accelerating the development of new therapeutic options.
Challenges and Considerations
While the potential benefits of RWD and RWE are considerable, there are a number of challenges that researchers and stakeholders must take into account. These include:
Data quality and standardization. RWD comes from a multitude of sources, leading to variations in data quality and format. Standardized collection and validation processes are essential for maintaining data accuracy.
Patient privacy and ethics. Protecting patient privacy, and ensuring ethical data usage and storage are critical. De-identifying data and obtaining informed consent are necessary steps in using RWD for research purposes.
Bias and confounding factors: Due to its real-world nature, RWD can reflect inherent biases. Researchers must employ advanced statistical methods to mitigate confounding factors and ensure the validity of their findings.
Data accessibility. Access to comprehensive and diverse datasets is essential for meaningful RWD analysis. Data-sharing among stakeholders, including healthcare institutions, researchers, and regulatory bodies, is critical.
Looking toward the future
The integration of RWD and RWE into clinical research is rapidly reshaping the way we understand healthcare interventions. As technology advances, wearable devices, mobile apps, and IoT devices are generating even more real-time data, providing researchers with a wealth of information to explore.
Additionally, advancements in machine learning and artificial intelligence make more sophisticated analysis techniques possible, enhancing our ability to extract meaningful insights from complex datasets.
Ultimately, RWD and RWE allow us to bridge the gap between controlled clinical trials and real-world patient outcomes, enabling a more comprehensive understanding of medical interventions.
While challenges exist, collaboration among researchers, clinicians, policymakers, and technology experts can pave the way for harnessing the full potential of real-world data and evidence to reflect the diversity and complexity of our healthcare landscape.

